Chemical tests test for fucntional groups and are vital in the organic lab
In CHEM 2274, the first test performed is the sodium fusion test, this test tests for halides and nitrogen.
The following material is quoted from:
Supplement to Laboratory Manual
(Fall 2009) Hagen J. and Wood J.
- Amines
- Hinsburg Test
- Diazo Coupling Test
- Nitrile
- Hydrolysis
- Carboxylic Acid
- Nutralization equivalent
- Acid Chloride
- Silver Nitrate
- Ester
- Hydroxamate
- β-Dicarbonyl
- Ferric Chloride Complex
- Halides and Nitrogen
Chemical Tests by functional group
- Alkenes and Alkynes
- Bromine Addition
- Bayer Test
- Aromatic Hydrocarbons
- Aluminum Chloride/Chloroform
- Halohydrocarbons
- Alcholoic Siver Nitrate Test
- Beilstein Flame Test
- Alcohols
- Ceric Ammonium Nitrate Test
- Lucas Test
- Chromic Acid Oxidation
- Phenols
- Ceric Ammonium Nitrate
- Ferric Chloride
- Aldehyde and Ketones
- 2,4-Dinitrophenylhydrazine
- Chromic Acid Oxidation
- Iodoform Formation
- Tollen's Test
- Schiff's Test
Alkenes and Alkynes
Bromine Addition
Add bromine and look for loss of bromine color
Bayer Test
Add potassium permanganate for loss of purple color
Aromatic Hydrocarbons
Aluminum Chloride/Chloroform
Add anhydrous aluminum chloride and look for bright colors indicating electrophilic aromatic substitution
Halohydrocarbons
Alcoloic Siver Nitrate Test
Add, ppt means halide present, yellow = bromine, purple = iodine, milky = chlorine
Beilstein Flame Test
Flame substance on copper wire, color indicates halides
Alcohols
Ceric Ammonium Nitrate Test
test
Lucas Test
test
Chromic Acid Oxidation
test
Phenols
Ceric Ammonium Nitrate
test
Ferric Chloride
Add ferric chloride and look for a red ppt.
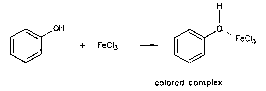
Aldehyde and Ketones
2,4-Dinitrophenylhydrazine
Aldehydes and Ketones (but not acids, esters, amides, anhydrides, and other carbonyl containing groups) react with 2,4-dinitrophenylhydrazine to form Shift's base derivatives (2,4- dinitrophenylhydrazones) as colored precipitates. A positive test is indicated by formation of a copious quantity of precipitate. Some alcohols contain small amounts of ketone or aldehyde impurities which will give a false positive test. Also, contamination of the test tube by acetone will give a false positive test.
Procedure -- In a large test tube add two drops (or half a microspatula) of the organic compound to 1 mL of ethanol. Once the substrate has dissolved, add 2-3 drops of the 2,4-DNP in H2SO4 reagent and shake the test tube vigorously. If no precipitate forms immediately, heat the solution in hot tap water for 1 min and then add five drops of water. The formation of a yellow to red precipitate is a positive test. Clean up: Organic waste container.
Chromic Acid Oxidation
Aldehydes are readily oxidized to acids by this reagent. Ketones are relatively unreactive
at room temperature. The reagent is reduced in the process to a blue-green precipitate of
chromium (III) sulfate. Any other colored precipitate is not a positive test. Aromatic
aldehydes react more slowly than aliphatic aldehydes; therefore, if no precipitate forms at once let
the tube stand for several minutes.
Other oxidizable groups like phenols, alcohols, alkenes, and alkynes give false positive
tests.
Procedure --In a large test tube add one drop of the organic compound to 2 mL of acetone and then add one drop of the chromic acid reagent. Note the time interval required for formation of the blue-green precipitate. Clean up: Acids/Metals waste container.
Iodoform Formation
look for carboxyl type ketones
Tollen's Test
use silver nitrate and make a silver mirror
Schiff's Test
test
Amines
Hinsburg Test
The Hinsberg test indicates whether a water insoluble amine is 1°, 2 °, or 3°. Primary amines give a sulfonamide derivative soluble in NaOH. Secondary amines give a base insoluble sulfonamide derivative. Water insoluble tertiary amines are inert (two layers would be seen). If a precipitate forms in the Hinsberg test, it can serve as a benzenesulfonamide derivative of your unknown amine. Alternatively, a larger quantity of a benzenesulfonamide derivative can be made by following the procedure in the "Preparation of Derivatives of an Unknown Amine" section below. If you know that your unknown is an amine, it will save you time to go to #2 below and follow the procedure on the larger scale.
Procedure -- In a large test tube, add 2 ml of methanol, five drops of the amine and eight drops of benzenesulfonyl chloride. Warm the mixture over a steam bath for at least 5 min with agitation. Add 2 mL of 6M aqueous sodium hydroxide. Cork the tube and then vigorously shake the solution for 10 min. Keep the mixtyie warm during this period in order to hydrolyze any excess benzenesulfonyl chloride that remains. Cool in an ice bath.
If the unknown amine is a 1° amine boiling less than 100°C, the mixture should be a homogeneous solution. Larger and higher boiling primary amines sometimes give salts insoluble in the concentrated base. If you get a solid at this point, see if it dissolves in water (cool then warm). If the unknown amine is a secondary amine, a precipitate of the sulfonamide derivative is usually observed in the test tube and it will NOT dissolve in water. If the amine is a water- insoluble tertiary amine, a layer of the unreacted amine is observed on the top of the aqueous solution.
If an precipitate formed in the basic solution, it can be collected by filtration and then washed twice with 5 mL of water. It is a sulfonamide derivative of a secondary amine.
If no precipitate appeared, add 6M HC1 (concentrated HC1 is 12M) slowly to the mixture until the solution is acidic. The sulfonamide of a 1° amine is insoluble in acidic solution. It can be collected by filtration, washed with 10% HC1 and then thoroughly with water to give the sulfonamide derivative.
If two layers were present, indicating an insoluble 3° amine, addition of 10% HC1 should give a homogeneous solution as the water soluble amine hydrochloride salt forms.
Clean up: Aqueous solutions of acids and bases are neutralized and put down the drain.
Diazo Coupling Test
test
Nitrile
Hydrolysis
test
Carboxylic Acid
Nutralization equivalent
test
Acid Chloride
Silver Nitrate
test
Ester
Hydroxamate
Esters can be converted into hydroxamic acids by reaction with hydroxylamine hydrochloride. The hydroxamic acid derivatives form red or violet complexes with aqueous ferric chloride. Phenols also give colored complexes with ferric chloride so an unknown compound must be tested first with ferric chloride before reaction with hydroxylamine to eliminate this possibility.
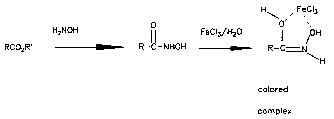
Procedure -- If 20 mg of the unknown compound shows no color change when treated with 0.5 mL of 5% aqueous ferric chloride, perform the rest of this procedure. To a clean test tube add 20 mg of the unknown compound tol mL of 1M hydroxylamine hydrochloride in ethanol. Then add dropwise a 15% ethanolic solution of potassium hydroxide until the test solution is just basic to pH test paper. Add five additional drops of 15% ethanolic potassium hydroxide. Heat the mixture to boiling for 30 seconds and then allow it to cool to room temperature. With thorough mixing, add dropwise an aqueous solution of 10% aqueous HC1 until the pH of the mixture is approximately 3. Add two drops of the 5% aqueous ferric chloride and note the color. The immediate formation of a red-blue or violet color is an indication that the original compound is an ester. Clean up: Place into the bottle marked "metals".
β-Dicarbonyl
Ferric Chloride Complex
test
Soduim Fusion Test
Some of the unknowns in this and subsequent experiments may contain nitrogen. Henceforth it will be necessary to test the sodium fusion filtrate for this element as well as for halides and to report the results on your cards and in your conclusions. While sodium fusion converts halogens into the corresponding halide ions, it converts nitrogen into the cyanide ion. The test for nitrogen is based upon the intense color produced when the cyanide ion adds to p- nitrobenzaldehyde.
Procedure -- Test for Halogens -- Place a wax coated pellet of sodium in a small pyrex test tube. Using tongs hold the tube over a flame to melt the wax and the sodium. Use the Bunsen burner only in the designated area. While still hot add two drops of the compound (10 mg of a solid) to the molten sodium. Heat the tube to red hot for 2 mins and then at once plunge the bottom into 10 mL of distilled water contained in a 50 mL beaker. Filter the solution by gravity into a large test tube. It should be colorless. A brown tinge will interfere with the later test for bromine. Remove 0.5 mL of the filtrate to test for the presence of C1-, Br-, or I- (F- is not detected). Use concentrated nitric acid to make the solution very acidic. Since nitrogen may be present in the filtrate, add a boiling stone and then boil the solution gently for two minutes to expel this element as HCN. Do this in the hood. Then add three drops of aqueous 5% AgNO3 (never use ethanolic AgNO3!). A white or light yellow precipitate indicates halide. A dark brown precipitate indicates that the pH is too high. Add more concentrated nitric acid. If this test is positive, continue.
To detect the specific halogen present, place 0.5 mL of the filtrate in a large test tube and then add 0.5 mL of CH2C12 as well as 3 drops of concentrated nitric acid. Shake carefully. A purple color indicates that iodide has oxidized to iodine. If no purple appears, go to *. If purple appears, remove the CH2C12 layer with a pipet. Add fresh CH2C12 and then shake to extract more iodine from the water layer. Again remove the bottom layer. Repeat this extraction process until the purple color is gone. The bottom CH2C12 layer should be colorless.
*Add 2 mL of concentrated nitric acid to the test tube and shake with care. A change from colorless to a faint brown color indicates that bromide has been oxidized to bromine. If no brown color appears, go to #. If brown color did appear, it must be extracted with CH2C12 as described above for iodine until the bottom layer is colorless.
#Finally, add three drops of 5% aqueous silver nitrate solution. A white precipitate is AgCl.
Clean up: Your test tube may have two layers (organic and aqueous) at this point. Put the aqueous supernatant layer down the drain. The methylene chloride layer must be put into the organic waste